Here’s A Quick Way To Solve A Tips About How To Choose A Solvent For Recrystallization

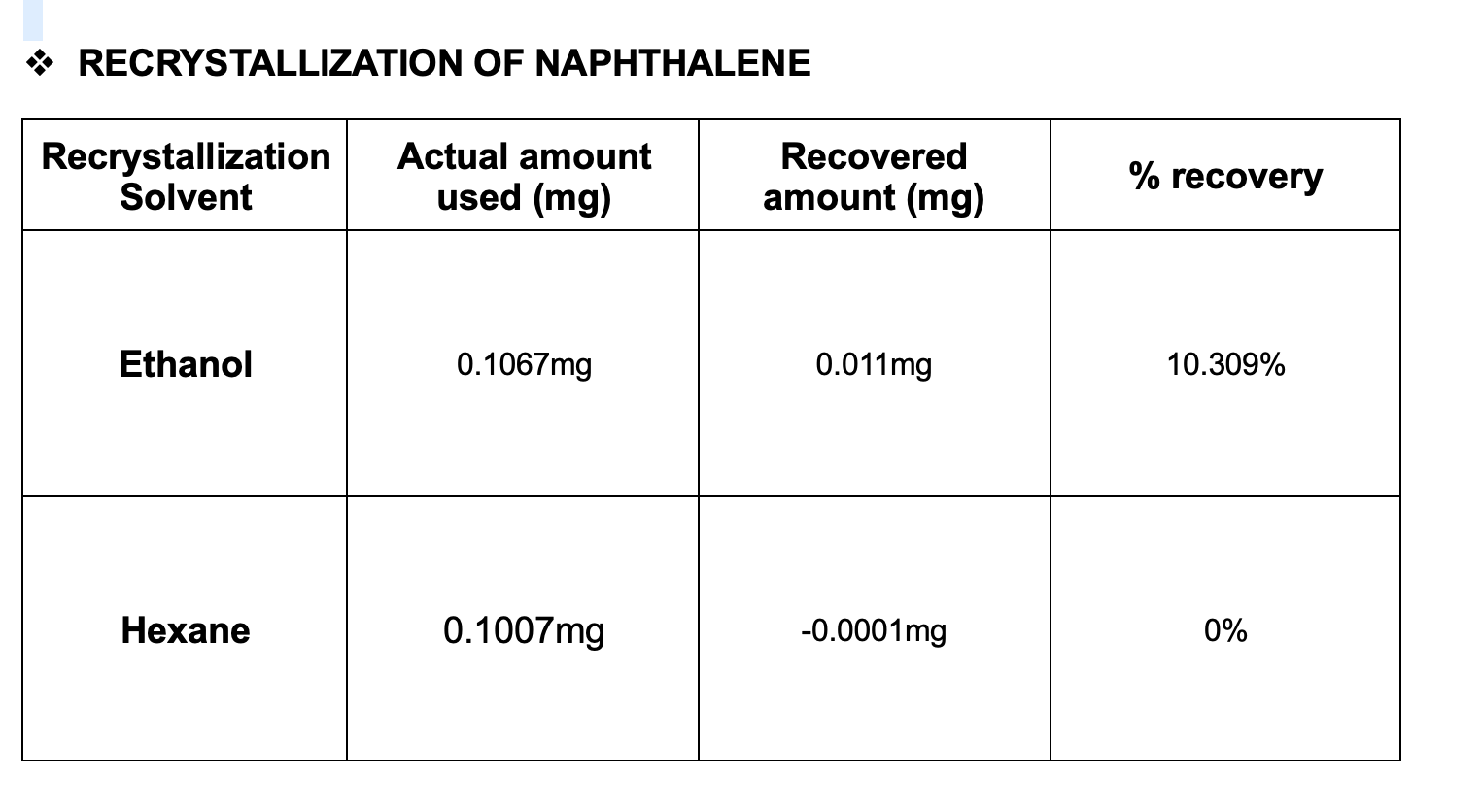
Recrystallization is used to purify solids.
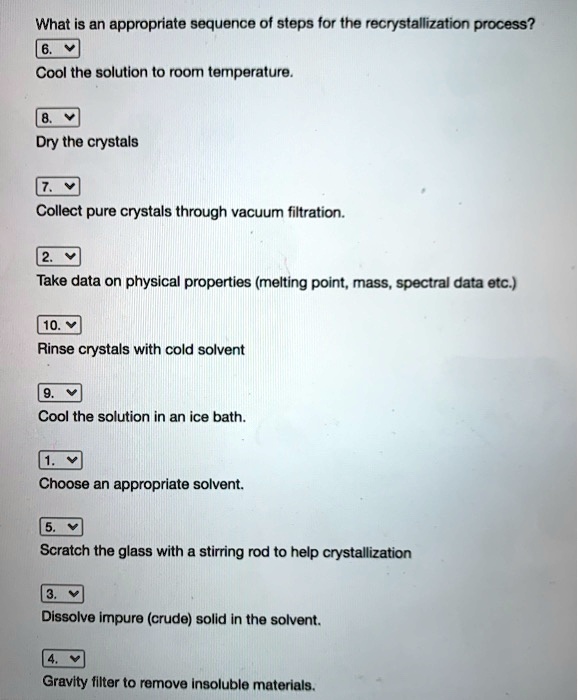
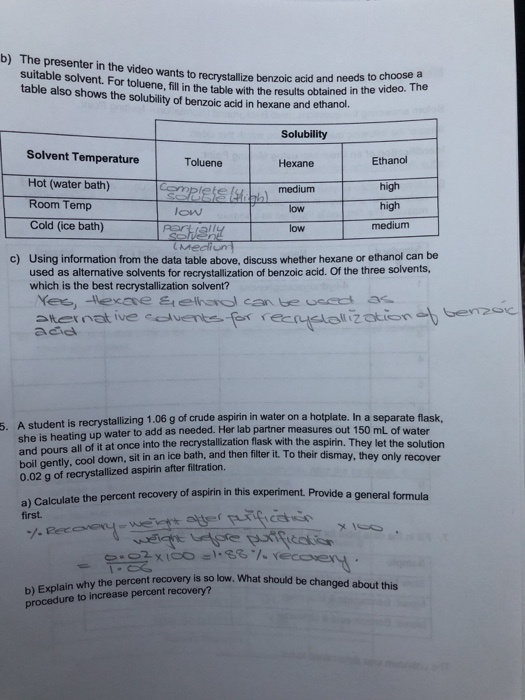
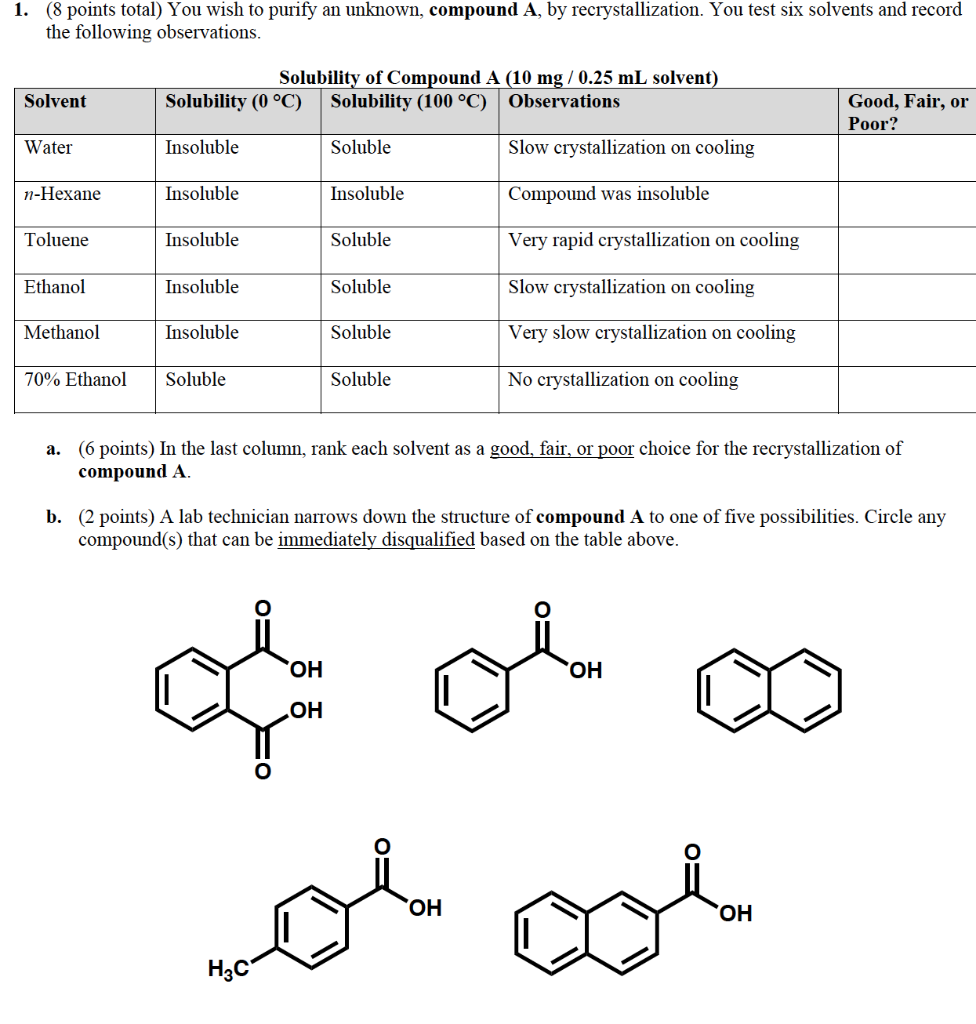
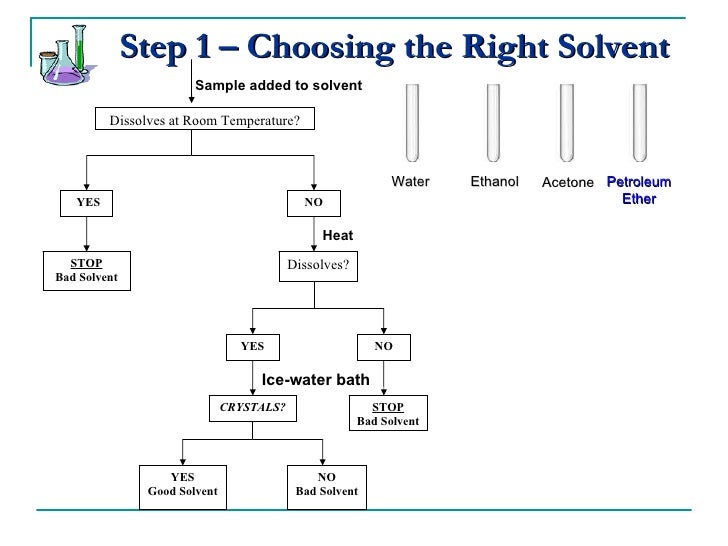
How to choose a solvent for recrystallization. There are five major steps in the recrystallization process: Besides having the crucial solubility properties for crystallization (the compound should be soluble in the hot solvent and as insoluble as possible in the cold solvent), there are other factors that determine an appropriate solvent. Refer to the flow chart shown.
The compounds must be more. The point is to fully. The first step of recrystallization is solvent selection.
Recrystallization solvent is one in which the solid has a very high solubility at high temperatures and a very low solubility at low temperatures. You have not visited any articles yet, please visit some articles to see contents here. The chosen recrystallization solvent will dissolve the compound when hot, but not at room temperature.
This is usually a combination of prediction/experience and trial/error. Types of recrystallization 1. The solvent must dissolve both.
A.) finding a solvent with a high temperature coefficient. Once again, you must choose the solvents very carefully. The crude impure solid is dissolved in hot solvent.
When no single solvent can be found that meets all of the criteria for crystallization, it may be possible to use a mixed solvent. The purpose of recrystallization is to separate or remove the impurities in a solid compound that are dissolved in a solvent to. Dissolving the solute in the solvent, performing a gravity filtration, if necessary, obtaining crystals of the solute,.
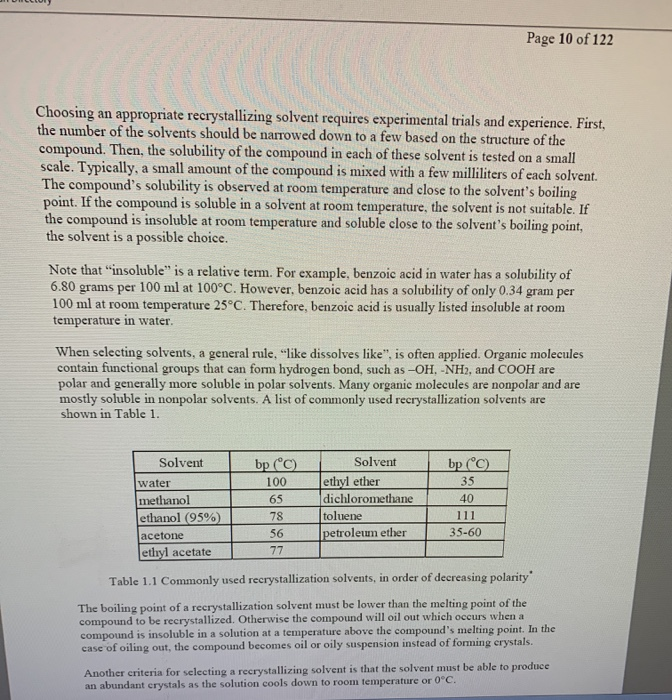
When one ideal solvent cannot be found for a crystallization, it is possible to use a mixture of two solvents. An ideal crystallization solvent should be. The criteria used to choose an appropriate recrystallization solvent includes:
An improved method of recrystallization. Use a stirring rod to agitate the solute or flick the bottom of the test tube with one finger while holding the top with the. 1 answer sorted by:
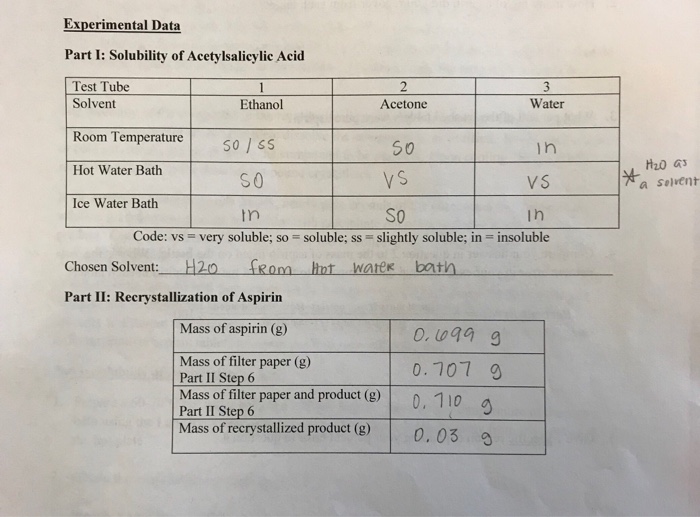
Choose a solvent such that the impure compound has poor solubility at low temperatures, yet is completely soluble at higher temperatures. In each test tube, place 0.5 ml of each potential solvent. If some solid remains undissolved after adding solvent, it is likely to be an impurity and.
17 generally speaking, the best solvent will be dependent on the impurity that you are trying to remove. The second solvent (solvent #2). Into five test tubes labeled with the names of the potential recrystallization solvents, place 0.10 gram of one of the solids you have chosen.